BIOLOGICS FOR RARE OPHTHALMIC CONDITIONS
Our focus is the advancement of product candidates based on our proprietary mesenchymal stem cell secretome (MSC-S) platform which we believe could address key unmet medical needs for rare corneal and retinal diseases.
Kala’s Mesenchymal Stem Cell Secretome (MSC-S) Platform Has the Potential to Address Multiple Unmet Clinical Needs in Ophthalmology via it’s Multifactorial Mechanism of Action
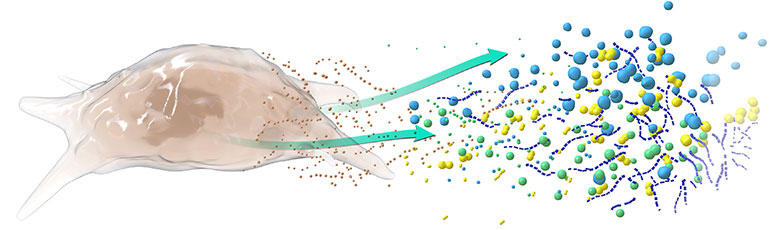
MSC-S Mechanisms of Action Include:
- Wound Healing/Tissue Repair
- Anti-inflammatory/Immunomodulatory
- Neurotrophic/Neuroprotective
The term “secretome” describes the biofactors secreted by cells into the extracellular space. In the case of our lead product KPI-012, these biofactors include classes of proteins such as matrix proteins, growth factors, neurotrophic factors and protease inhibitors that are relevant to corneal wound-healing. Secretomes are cell-free and have emerged as an attractive treatment for numerous unmet medical needs in ophthalmology and other branches of medicine as they may provide the multifactorial benefits of traditional cell therapy while overcoming many of their translational barriers (Ahangar et al., 2020; Visozo et al., 2017).
See corporate overview for more information (opens a PDF document in a new window)Kala’s MSC-S platform is an allogenic therapy based on a cell bank created from a human bone-marrow derived mesenchymal stem cell source. The manufacturing process for KPI-012 is comprised of three stages: (1) cultivation of mesenchymal stem cells from a working cell bank and production of unprocessed conditioned media (cell-free secretome), (2) production of drug substance as a chemically defined solution, and (3) formulation and filling of drug product.
KPI-012: A Novel Clinical Stage Mesenchymal Stem Cell Secretome (MSC-S) Therapy in Development for Multiple Rare Ocular Surface Diseases
Our lead clinical-stage candidate is KPI-012, a topically dosed ophthalmic therapy developed from our MSC-S platform. KPI-012 is currently in clinical development for the treatment of persistent corneal epithelial defect, or PCED, a rare disease of impaired corneal healing. Based on the positive results of a Phase 1b clinical efficacy trial of KPI-012 in patients with PCED, on November 28, 2022 we announced the submission of an investigational new drug (IND) application to the U.S. Food and Drug Administration (FDA) for KPI-012 for the treatment of Persistent Corneal Epithelial Defect (PCED). Subject to acceptance of the IND by the FDA, Kala remains on-track to initiate a Phase 2b clinical trial of KPI-012 for PCED in the fourth quarter of 2022. Topline safety and efficacy data from the trial is expected in the first quarter of 2024. If positive, this trial could serve as the first of two pivotal trials needed to support the submission of a Biologics License Agreement (BLA) to the FDA. KPI-012 has received orphan drug designation from the FDA for the treatment of PCED.
See corporate overview for more information (opens a PDF document in a new window)KPI-012 contains multiple classes of proteins relevant to ocular surface tissue repair, including matrix proteins, growth factors, neurotrophic factors and protease inhibitors. We believe that the presence of these factors enables KPI-012 to provide a multifactorial mechanism of action that is relevant to the treatment of multiple rare ocular surface diseases. In addition to PCED, we are planning to advance KPI-012 for the treatment of partial limbal stem cell deficiency and ocular manifestations of moderate-to-severe Sjögren's.
KPI-014: Our MSC-S Preclinical Program in Inherited Retinal Disease
We believe that our MSC-S platform has the potential to provide a genotype-agnostic treatment for one or more inherited retinal diseases by functionally enhancing and reducing degeneration of the relevant retinal cell types. Independent investigators studying secretome derived from other mesenchymal stem cell sources have demonstrated its neuroprotective effect in both in vitro and in vivo models of retinal degeneration by several critical mechanisms, including antioxidant modulation and inhibition of apoptosis (Usategui-Martín, et. al., 2020, 2021 and 2022 and Adak et al., 2021). Biologically active components we are able to produce from our proprietary MSC-S platform include neurotrophic, growth, anti-inflammatory/immune-modulatory, and antioxidant factors. We plan to select a retinal indication in the second half of 2023 based on pre-clinical data read out.
Bibliography
- Adak S, Magdalene D, Deshmukh S, et al. A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem Cell Rev Rep. 2021. Aug;17(4):1154-1173.
- Vizoso FJ, Eiro N, Cid S, et al. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017. Aug 25; 18(9):1852.
- Usategui-Martín R, Puertas-Neyra K, García-Gutiérrez MT, et al. Human Mesenchymal Stem Cell Secretome Exhibits a Neuroprotective Effect over In Vitro Retinal Photoreceptor Degeneration. Mol Ther Methods Clin Dev. 2020. May 13;17:1155-1166.
- Usategui-Martín R and Fernández-Bueno I. Neuroprotective therapy for retinal neurodegenerative diseases by stem cell secretome. Neural Regeneration Research. 2021(16): 117-118.
- Usategui-Martín R, Puertas-Neyra K, Galindo-Cabello N, et al. Retinal Neuroprotective Effect of Mesenchymal Stem Cells Secretome Through Modulation of Oxidative Stress, Autophagy, and Programmed Cell Death. Investigative Ophthalmology & Visual Science. 2022. Apr;63(4):27.