

Fulfilling the promise of mesenchymal stem cell secretome (MSC-S) therapy for rare eye diseases.
KALA BIO is developing treatments that focus the power of our MSC-S platform on significant unmet needs across a range of rare ophthalmic diseases.
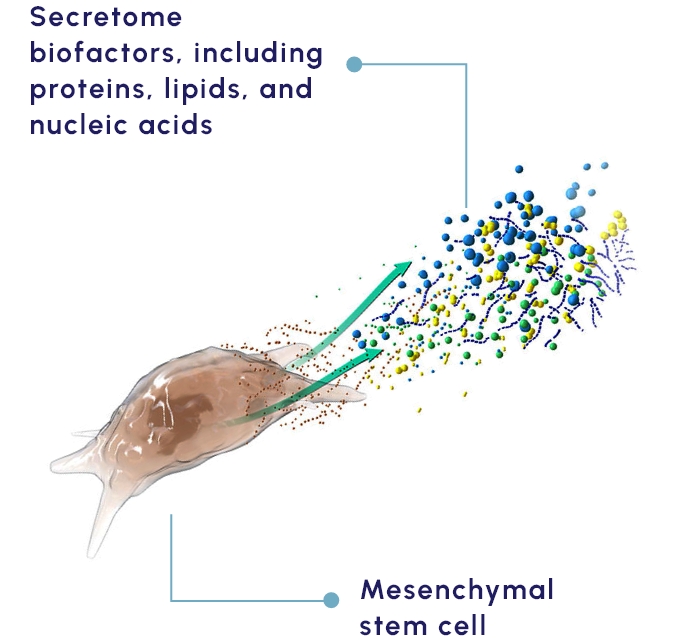
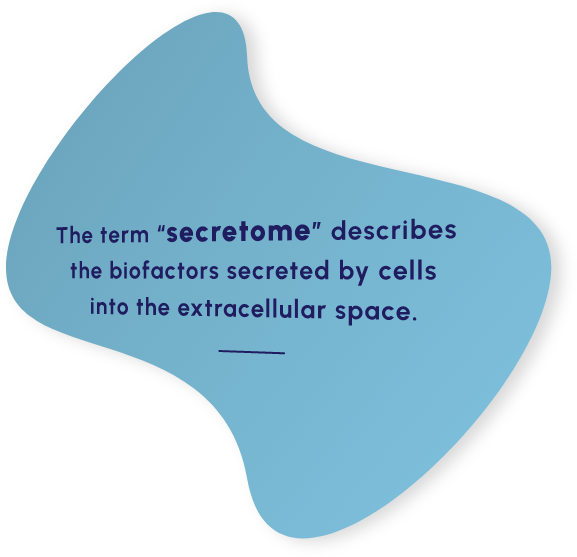
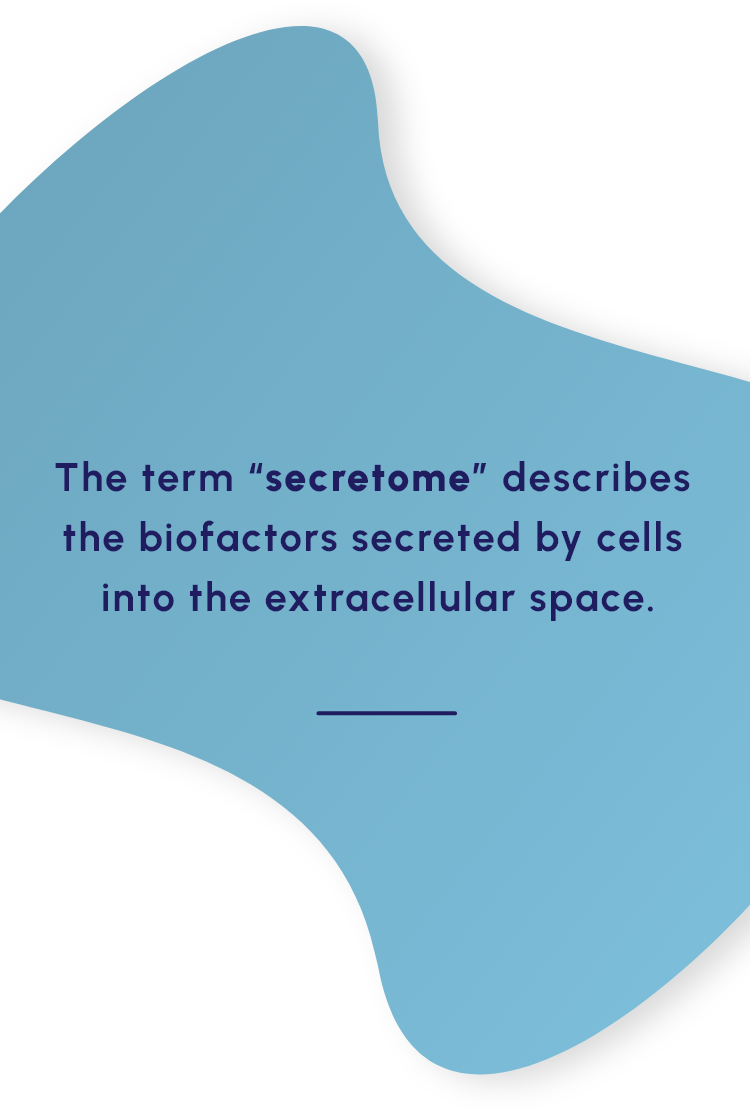
Our proprietary MSC-S platform is a cell-free approach to disease management that utilizes the regenerative potential of human MSC-S to drive the therapeutic benefit. The secretome provides a multifactorial approach shown to promote beneficial response in preclinical and clinical studies that include:
KPI-012, our secretome-based lead product candidate, contains several classes of human proteins shown in preclinical studies to promote ocular surface tissue repair.
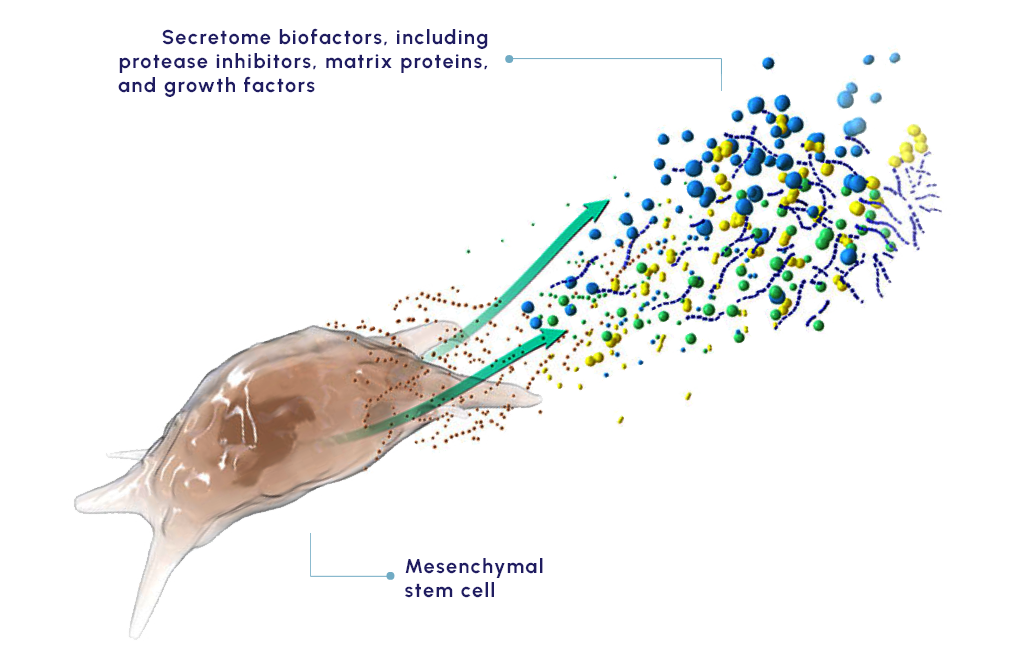
Secretomes offer the benefits of cell therapy while avoiding many of the safety and logistical concerns of implanting cells.
Secretomes offer the benefits of cell therapy while avoiding many of the safety and logistical concerns of implanting cells.
KPI-012 is manufactured under GMP utilizing a proprietary human cell bank and well-defined CMC process, enabling consistent lot-to-lot stability and biopotency.
The manufacturing
process for our
innovative products includes 3 stages:
includes 3 stages:
-
1
Expansion
of human mesenchymal stem cells from a working cell bank and production of unprocessed cell-free secretome. -
2
Production
of purified drug substance. -
3
Formulation
and filling of drug product.
CMC, Chemistry, Manufacturing, and Controls; GMP, Good Manufacturing Practices.





